Embark on a captivating exploration of acids, bases, and pH with our comprehensive Acids/Bases & pH Worksheet Answer Key. Delve into the fundamental concepts, unravel the intricacies of the pH scale, and master the art of acid-base reactions. Our meticulously crafted guide empowers you to navigate the complexities of chemical interactions with clarity and confidence.
From the definitions of acids and bases to the practical applications of buffers, this answer key serves as your trusted companion, providing in-depth explanations, illustrative examples, and expert insights. Prepare to enhance your understanding of these essential chemical principles and excel in your academic pursuits.
Acid-Base Concepts
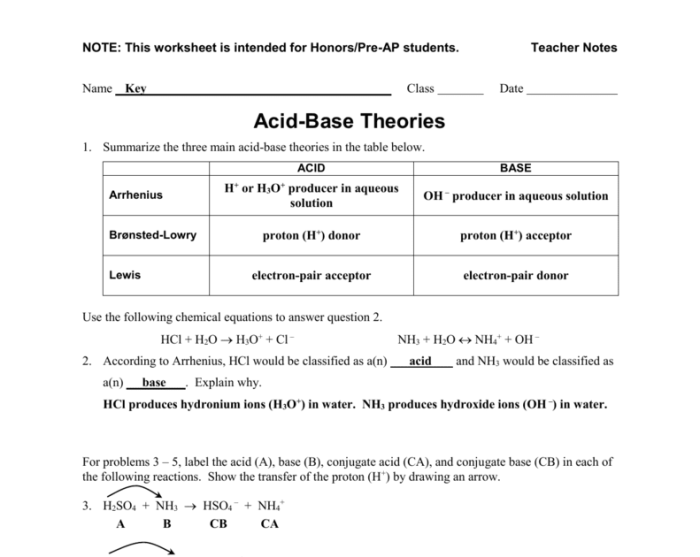
In chemistry, acids and bases are two important concepts that describe the chemical properties of substances. According to the Arrhenius theory, an acid is a substance that, when dissolved in water, produces hydrogen ions (H+). A base, on the other hand, is a substance that, when dissolved in water, produces hydroxide ions (OH-).
Acids and bases have distinct properties. Acids are typically sour in taste, corrosive to skin, and react with metals to produce hydrogen gas. Bases are typically bitter in taste, slippery to the touch, and react with acids to produce salts and water.
Common Acids and Bases
There are many common acids and bases. Some of the most common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Some of the most common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
pH Scale
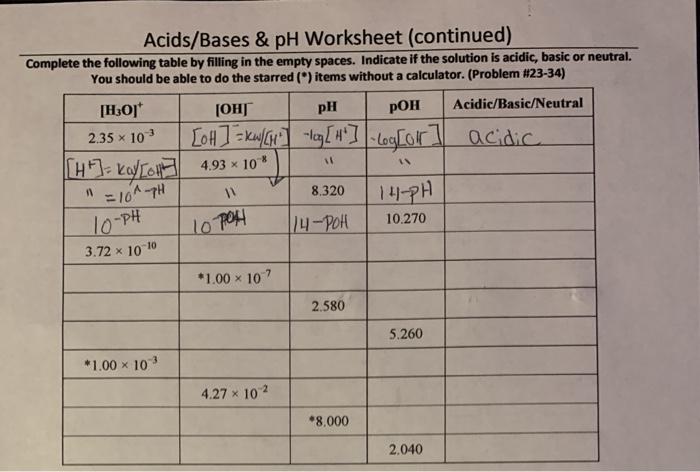
pH is a measure of the acidity or alkalinity of a solution. It is defined as the negative logarithm (base 10) of the hydrogen ion concentration in moles per liter.
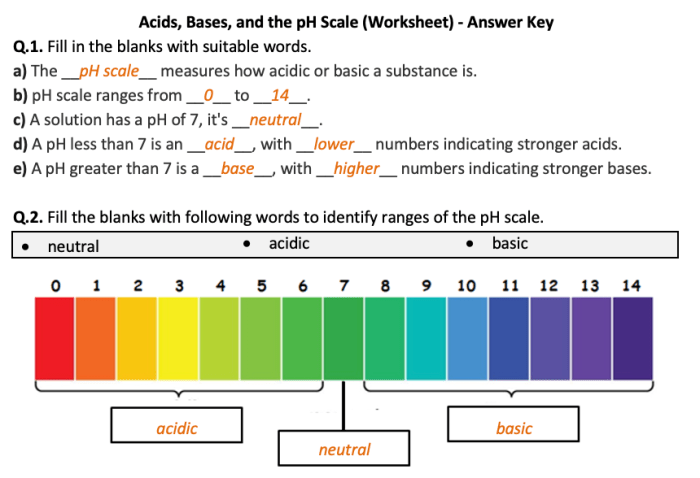
The pH scale ranges from 0 to 14. A pH of 7 is neutral. Values below 7 are acidic, while values above 7 are alkaline (or basic).
Relationship Between pH and Hydrogen Ion Concentration, Acids/bases & ph worksheet answer key
The pH of a solution is inversely proportional to the hydrogen ion concentration. This means that as the hydrogen ion concentration increases, the pH decreases. Conversely, as the hydrogen ion concentration decreases, the pH increases.
pH =
log[H+],
where [H+] is the hydrogen ion concentration in moles per liter.
Acid-Base Reactions
Acid-base reactions are chemical reactions that involve the transfer of protons (H+ ions) between reactants. These reactions play a crucial role in various chemical and biological processes, including digestion, photosynthesis, and industrial processes.
There are three main types of acid-base reactions:
Neutralization Reactions
Neutralization reactions occur when an acid and a base react in stoichiometric proportions to form a salt and water. The products of a neutralization reaction are typically neutral, meaning they have a pH of 7.
- Example: HCl + NaOH → NaCl + H2O
Precipitation Reactions
Precipitation reactions occur when two solutions containing ions react to form an insoluble solid precipitate. Precipitation reactions are often used to separate and purify substances.
- Example: AgNO3 + NaCl → AgCl(s) + NaNO3
Gas Evolution Reactions
Gas evolution reactions occur when an acid reacts with a metal or a carbonate to produce a gas. Gas evolution reactions are often used to generate gases for industrial or laboratory purposes.
- Example: HCl + Zn → ZnCl2 + H2
- Example: HCl + Na2CO3 → NaCl + H2O + CO2
Acid-Base Titration: Acids/bases & Ph Worksheet Answer Key
Acid-base titration is a laboratory technique used to determine the concentration of an unknown acid or base. It involves the controlled addition of a known volume of a solution with a known concentration (the titrant) to a solution with an unknown concentration (the analyte) until the reaction between the two solutions is complete.
The point at which the reaction is complete is called the equivalence point.
Steps Involved in Acid-Base Titration
- The analyte solution is placed in a flask.
- A few drops of an indicator are added to the analyte solution.
- The titrant is added slowly from a burette to the analyte solution while swirling the flask.
- The color of the indicator changes when the equivalence point is reached.
- The volume of titrant added is recorded.
Calculating the Concentration of an Unknown Acid or Base
The concentration of the unknown acid or base can be calculated using the following formula:
M1V 1= M 2V 2
where:
- M 1is the concentration of the titrant (known)
- V 1is the volume of titrant added (recorded)
- M 2is the concentration of the analyte (unknown)
- V 2is the volume of analyte used
By rearranging the formula, the concentration of the analyte can be calculated as follows:
M2= (M 1V 1) / V 2
Acid-Base Buffers
Acid-base buffers are solutions that resist changes in pH when small amounts of acid or base are added to them. They play a vital role in maintaining the pH of various biological systems, such as blood and the intracellular environment, within a narrow range necessary for optimal functioning.
Mechanism of Buffer Action
Buffers consist of a weak acid and its conjugate base or a weak base and its conjugate acid. When a small amount of acid is added to a buffer, the weak acid reacts with the hydrogen ions, preventing a significant increase in pH.
Conversely, when a small amount of base is added, the conjugate base reacts with the hydroxide ions, preventing a significant decrease in pH.
Common Buffer Systems
- Carbonic Acid-Bicarbonate Buffer:Found in blood and other bodily fluids, it consists of carbonic acid (H 2CO 3) and bicarbonate ion (HCO 3–).
- Phosphoric Acid-Dihydrogen Phosphate Buffer:Used in biochemistry and molecular biology, it consists of phosphoric acid (H 3PO 4) and dihydrogen phosphate ion (H 2PO 4–).
- Acetic Acid-Acetate Buffer:Commonly used in chemistry and industry, it consists of acetic acid (CH 3COOH) and acetate ion (CH 3COO –).
Applications of Acids and Bases
Acids and bases have numerous applications in various fields, including food, medicine, and industry. They play crucial roles in biological systems and have environmental implications.
In Food
- Acids are used as preservatives in food to prevent spoilage by inhibiting the growth of microorganisms.
- Bases are used as leavening agents in baking to produce carbon dioxide gas, which causes dough to rise.
- Acids and bases are also used to enhance flavor and adjust the pH of food products.
In Medicine
- Acids are used in medications to treat indigestion, heartburn, and other stomach ailments.
- Bases are used in antacids to neutralize stomach acid.
- Acids and bases are also used in various medical tests and procedures.
In Industry
- Acids are used in the production of fertilizers, dyes, and other chemicals.
- Bases are used in the production of soaps, detergents, and paper.
- Acids and bases are also used in various industrial processes, such as metalworking and water treatment.
In Biological Systems
Acids and bases play vital roles in biological systems. The pH of body fluids, such as blood and urine, is tightly regulated to maintain homeostasis.
Acids are involved in digestion, breaking down food into smaller molecules. Bases are involved in the transport of oxygen in the blood.
Environmental Implications
Acid rain is a major environmental problem caused by the release of sulfur dioxide and nitrogen oxides into the atmosphere. These gases react with water vapor to form acids, which can damage forests, lakes, and buildings.
Query Resolution
What is the difference between an acid and a base?
According to the Arrhenius theory, an acid is a substance that produces hydrogen ions (H+) when dissolved in water, while a base is a substance that produces hydroxide ions (OH-) when dissolved in water.
What is the pH scale?
The pH scale is a measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while solutions with a pH above 7 are basic.
What is the purpose of acid-base titration?
Acid-base titration is a technique used to determine the concentration of an unknown acid or base. It involves adding a known volume of a base to an unknown volume of acid until the solution reaches a neutral point, as indicated by a pH indicator.