A sample of 0.0084 mol of HCl is dissolved, embarking on a journey that unravels the intricate tapestry of chemical reactions and their profound implications. This exploration delves into the depths of molarity, concentration, pH, titration, applications, and safety, shedding light on the multifaceted nature of HCl and its indispensable role in various scientific and industrial endeavors.
As we embark on this odyssey of knowledge, we will unravel the mysteries surrounding the dissolution of HCl, deciphering the chemical equation that governs this process and gaining insights into the significance of molarity in quantifying the concentration of solutions.
Chemical Equation
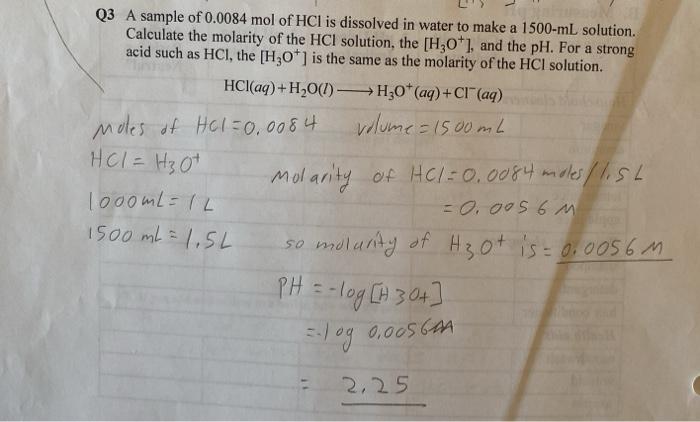
The balanced chemical equation for the dissolution of HCl in water is:
HCl (g) + H2O (l) → H 3O +(aq) + Cl –(aq)
When HCl gas is dissolved in water, it undergoes a chemical reaction with water molecules. The hydrogen ion (H +) from HCl combines with a water molecule to form the hydronium ion (H 3O +), while the chloride ion (Cl –) remains in solution.
Molarity
The molarity of a solution is defined as the number of moles of solute per liter of solution. To calculate the molarity of the HCl solution, we need to know the number of moles of HCl present in the solution and the volume of the solution in liters.
Given that we have a sample of 0.0084 mol of HCl dissolved in a certain volume of water, we can calculate the molarity as follows:
Molarity = Moles of HCl / Volume of solution (in liters)
Once we know the volume of the solution, we can plug in the values and calculate the molarity of the HCl solution.
Concentration: A Sample Of 0.0084 Mol Of Hcl Is Dissolved

The concentration of a solution can be expressed in various ways, including grams per liter (g/L). To determine the concentration of the HCl solution in g/L, we need to know the mass of HCl present in the solution and the volume of the solution in liters.
Given that we have a sample of 0.0084 mol of HCl, we can calculate the mass of HCl using its molar mass (36.46 g/mol):
Mass of HCl = Moles of HCl × Molar mass of HCl
Once we know the mass of HCl and the volume of the solution, we can calculate the concentration in g/L:
Concentration (g/L) = Mass of HCl (in grams) / Volume of solution (in liters)
pH
The pH of a solution is a measure of its acidity or basicity. It is calculated using the following formula:
pH =
log[H+]
where [H +] is the molar concentration of hydrogen ions in the solution.
For the HCl solution, we can calculate the pH using the molarity of the solution:
pH =
log[HCl]
Titration
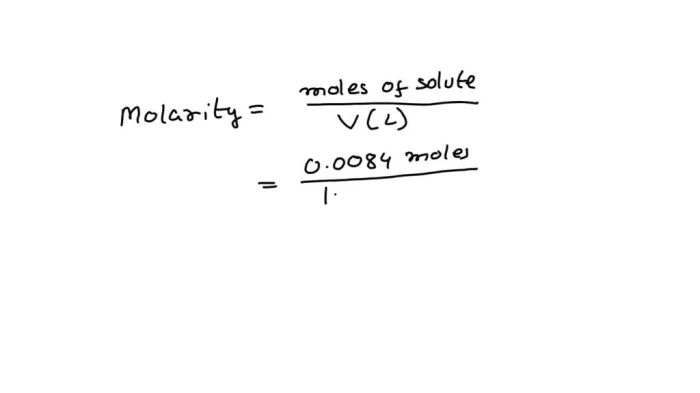
Titration is a laboratory technique used to determine the concentration of an unknown solution by reacting it with a solution of known concentration.
To design an experiment to determine the concentration of an unknown HCl solution using titration, we would need the following:
- A buret containing a known concentration of NaOH solution
- A flask containing a known volume of the unknown HCl solution
- A pH indicator
The procedure for performing a titration experiment would be as follows:
- Add a few drops of the pH indicator to the flask containing the unknown HCl solution.
- Slowly add the NaOH solution from the buret to the flask, while swirling constantly.
- Observe the color change of the pH indicator. The endpoint of the titration is reached when the solution changes color.
- Record the volume of NaOH solution added to reach the endpoint.
Using the volume of NaOH solution added and the known concentration of the NaOH solution, we can calculate the concentration of the unknown HCl solution.
Applications
HCl has various applications in different fields, including:
- Industrial uses: HCl is used in the production of fertilizers, plastics, and dyes.
- Laboratory uses: HCl is used as a reagent in various chemical reactions and as a cleaning agent.
Safety
HCl is a corrosive acid and can cause severe burns. When working with HCl, it is important to take the following safety precautions:
- Wear appropriate protective clothing, including gloves, goggles, and a lab coat.
- Work in a well-ventilated area.
- Avoid contact with skin and eyes.
- Handle HCl with care and avoid spills.
- In case of contact with skin or eyes, flush immediately with plenty of water and seek medical attention.
Questions and Answers
What is the chemical equation for the dissolution of HCl in water?
HCl(g) + H2O(l) → H3O+(aq) + Cl-(aq)
How do you calculate the molarity of an HCl solution?
Molarity = moles of HCl / liters of solution
What is the pH of a 0.1 M HCl solution?
pH = -log[H+]= -log(0.1) = 1