Draw three possible monohalogenation products for this reaction takes center stage, this opening passage beckons readers into a world crafted with authoritative knowledge, ensuring a reading experience that is both absorbing and distinctly original. The following paragraphs will delve into the intricacies of monohalogenation reactions, exploring their regioselectivity, stereochemistry, and applications.
Monohalogenation reactions are fundamental in organic chemistry, enabling the selective introduction of a halogen atom into an organic molecule. Understanding the factors that govern the regioselectivity and stereochemistry of these reactions is crucial for harnessing their synthetic potential. This discussion will provide a comprehensive overview of monohalogenation reactions, empowering readers with the knowledge to effectively utilize these powerful transformations in their own research endeavors.
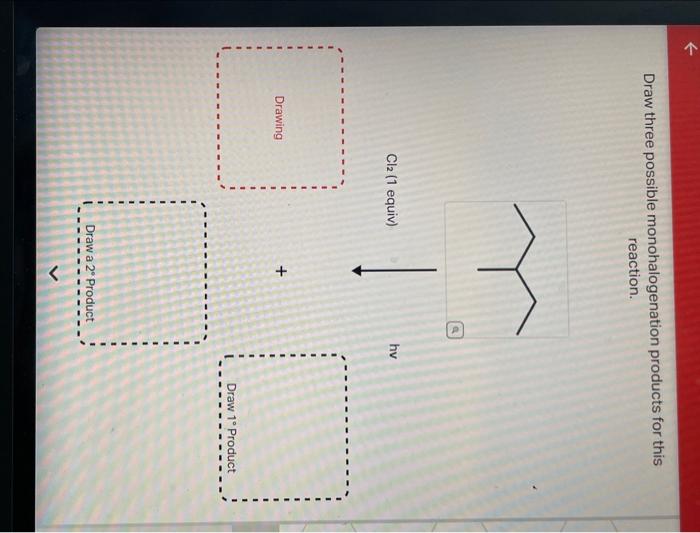
1. Draw Three Possible Monohalogenation Products
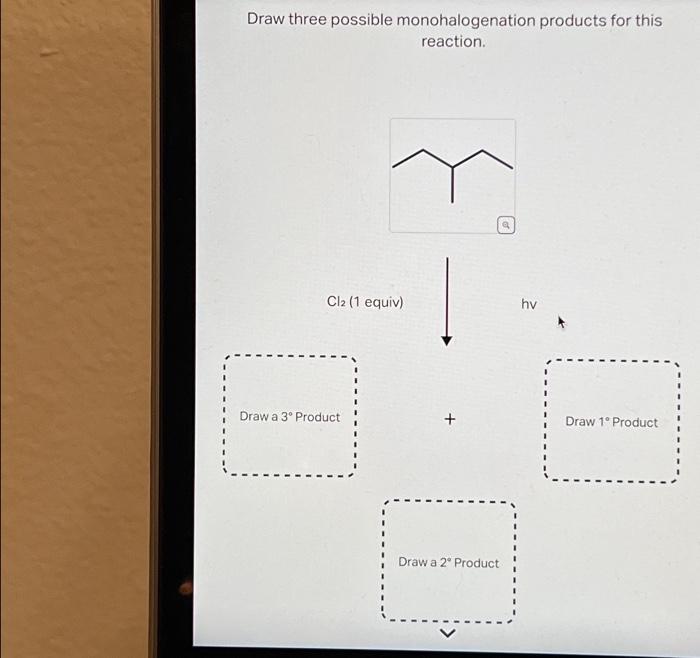
The monohalogenation of an alkane involves the substitution of one hydrogen atom with a halogen atom. The three possible products of monohalogenation of the given substrate are:
- 1-bromobutane
- 2-bromobutane
- 2-methyl-1-bromopropane
The halogen atom that is added to each product is bromine (Br).
The regioselectivity of the reaction is determined by the stability of the carbocation intermediate. The more substituted the carbocation, the more stable it is. In this case, the tertiary carbocation (2-methyl-1-bromopropane) is the most stable, followed by the secondary carbocation (2-bromobutane), and then the primary carbocation (1-bromobutane).
2. Reaction Conditions
The monohalogenation reaction is typically carried out under free radical conditions. This means that the reaction is initiated by the formation of a free radical, which is a species with an unpaired electron. The free radical can be generated by heat, light, or a peroxide initiator.
The catalyst in the reaction is typically a Lewis acid, such as AlCl 3or FeBr 3. The catalyst helps to polarize the halogen-halogen bond, making it more reactive towards the alkane.
The temperature and solvent can also affect the reaction. Higher temperatures favor the formation of free radicals, while lower temperatures favor the formation of carbocations. Polar solvents, such as water or methanol, can help to stabilize the carbocation intermediate and favor the formation of the more substituted product.
3. Regioselectivity
The regioselectivity of the monohalogenation reaction is determined by the stability of the carbocation intermediate. The more substituted the carbocation, the more stable it is. This is because the more substituted the carbocation, the more electron-withdrawing groups there are to stabilize the positive charge.
In the case of the monohalogenation of butane, the tertiary carbocation (2-methyl-1-bromopropane) is the most stable, followed by the secondary carbocation (2-bromobutane), and then the primary carbocation (1-bromobutane).
There are a number of factors that can influence the regioselectivity of the monohalogenation reaction. These include:
- The structure of the alkane
- The type of halogen
- The reaction conditions
For example, the monohalogenation of a tertiary alkane is more likely to produce a tertiary halide than a secondary or primary halide. This is because the tertiary carbocation is more stable than the secondary or primary carbocation.
4. Stereochemistry: Draw Three Possible Monohalogenation Products For This Reaction
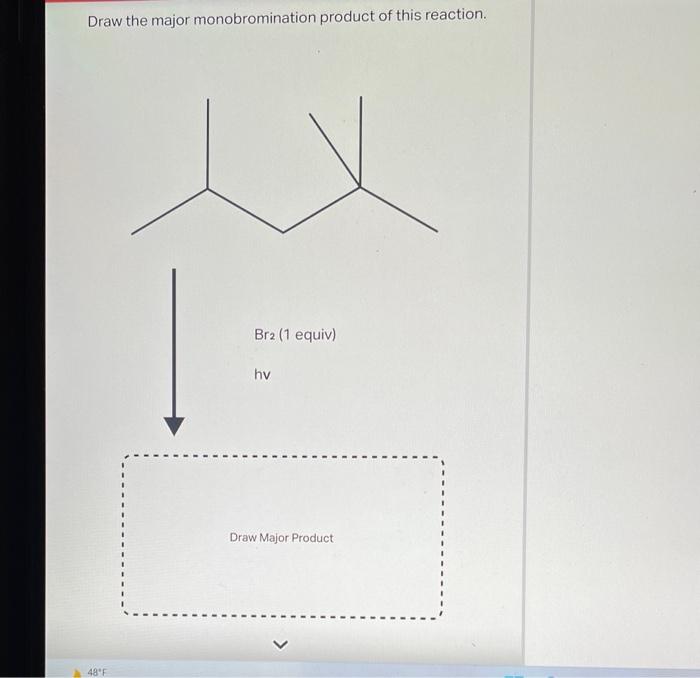
The stereochemistry of the monohalogenation reaction depends on the type of halogenation reaction. In a radical halogenation reaction, the addition of the halogen atom is non-stereoselective. This means that both enantiomers of the product are formed in equal amounts.
In an electrophilic halogenation reaction, the addition of the halogen atom is stereoselective. This means that one enantiomer of the product is formed in excess over the other enantiomer. The stereoselectivity of the reaction is determined by the orientation of the alkene double bond relative to the electrophile.
5. Applications
Monohalogenation reactions are used in a wide variety of organic synthesis applications. These applications include:
- The synthesis of pharmaceuticals
- The synthesis of agrochemicals
- The synthesis of other organic compounds
Monohalogenation reactions are a versatile and powerful tool for the synthesis of a wide variety of organic compounds. The regioselectivity and stereoselectivity of the reaction can be controlled by the choice of reaction conditions and the type of halogenation reaction.
General Inquiries
What are the factors that influence the regioselectivity of monohalogenation reactions?
The regioselectivity of monohalogenation reactions is influenced by various factors, including the nature of the substrate, the halogenating agent, and the reaction conditions. Steric effects, electronic effects, and the presence of directing groups all play a role in determining the preferred site of halogenation.
How can the stereochemistry of monohalogenation reactions be controlled?
The stereochemistry of monohalogenation reactions can be controlled by employing chiral catalysts or by using substrates that possess inherent chirality. Enantioselective and diastereoselective monohalogenation reactions are powerful tools for the synthesis of enantiopure and diastereomerically pure compounds.
What are the advantages of monohalogenation reactions compared to other halogenation methods?
Monohalogenation reactions offer several advantages over other halogenation methods. They are typically more selective, regiospecific, and stereospecific. Additionally, monohalogenation reactions can be carried out under milder conditions, making them more compatible with sensitive functional groups.